Cubic lipid structures are found in the plasma membrane of thermoacidophilic archaebacteriae as infoldings of the respective lipid membranes [1, 2] and further in subcellular structures of eucaryotic cells like the endoplasmatic reticulum, nucleus, or mitochondria [3]. By now little is known about the possible functional relevance of these topological changes in the membrane structures, which seem to play an important role in dynamic processes.
Total lipid extracts of Escherichia coli are rich of non-lamellar forming lipids and form inverse bicontinuous cubic phases of the space group Pn3m at elevated temperatures. These cubic phases, exhibiting periodic minimal surfaces, consist of curved membranes that fill up space in a regular pattern, forming a complicated network of interconnected water tubes. This study aimed to gain information on the effect of membrane-active compounds like antimicrobial amphipathic peptides on the formation of the inverse bicontinuous cubic phases.
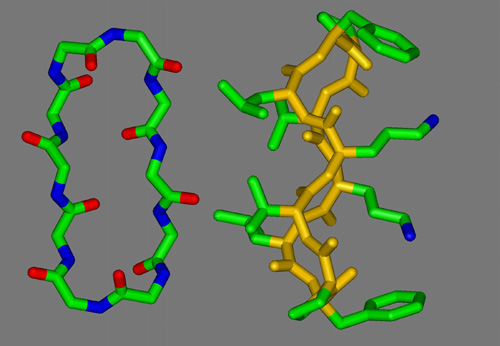
Mixtures of E. coli lipid extracts and membrane-active peptides (lipid-to-peptide molar ratio of 25:1) were measured at 25 deg C using rotating capillaries. The small-angle X-ray scattering experiments carried out at the SAXS-beamline at ELETTRA (Trieste, Italy) clearly showed the formation of cubic phases, predominantly of space group Pn3m. In the presence of gramicidin S, a cyclic decapeptide from Bacillus braevis (Fig.1), the lattice spacing was reduced from 14.9 to 13.4 nm (Fig.2) as compared to the pure lipid, whereas the other membrane-active peptides studied, i.e. GS14 (the 14-mer analogous peptide of gramicidin S), the frog skin peptide, PGLa, and PG-1 from porcine leukocytes, lead to a significant increase of the cubic lattice (16.7 nm, GS14; 17.4 nm, PGLa; 17.2 nm, PG-1). Further, the hkl-reflections corresponding to a cubic phase of space group Pn3m were much less resolved for mixtures containing PGLa (a -helical structure) or PG-1 (b -sheet conformation). This observation may indicate a lower preference of the non-cyclic peptides for promotion of cubic lipid phases. This work has been published recently [4] and will be the basis for future experiments, where the influence of temperature, ionic strength and in particular lipid composition on the formation/promotion of inverse bicontinuous cubic lipid phases will be studied in more detail.

Figure 1. Structure and conformation of gramicidin S. Left: view perpendicular to the plane of the ring, illustrating the peptide backbone structure. The antiparallel b -sheet region is stabilized by hydrogen bonds. Right: side-view, indicating the disposition in space of the hydrophobic Val and Leu residues (left) and the basic Orn (right) relative to the peptide ring.
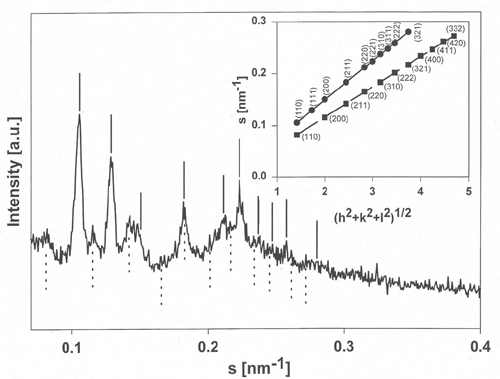
Figure 2. Small-angle X-ray diffractogram of a mixture of E. coli membrane total lipid extract and gramicidin S (lipid-to-peptide molar ratio of 25:1) recorded at 25 deg C. Position of the hkl-reflections corresponding to a cubic phase of space group Pn3m (solid line) and Im3m (dotted line) are indicated. Insert: Indexing of the two cubic lattices from the plot s, the reciprocal scattering vector, vs. (h2 + k2 + l2)1/2.